You’ll frequently encounter plastic pollution referred to as an issue. However, the truth is that it comprises multiple issues. Depending on the characteristics we seek, we create plastics from various polymers, each bound together by a unique type of chemical bond. Therefore, the technique we utilize to decompose one form of polymer might be unsuitable for the chemistry of another.
This is the issue, which is why, despite having made advances in discovering enzymes that decompose prevalent plastics like polyesters and PET, they remain only partial solutions to plastic waste. Nonetheless, researchers are not complacent with the success of these partial solutions; they now possess highly advanced protein design tools to assist them.
This is the narrative behind a brand-new enzyme that researchers have created to decompose polyurethane, the polymer frequently employed in foam cushioning production, among other uses. The novel enzyme is suitable for an industrial-grade recycling process that disassembles the polymer into its fundamental building blocks, which can be utilized to produce new polyurethane.
Decomposing polyurethane
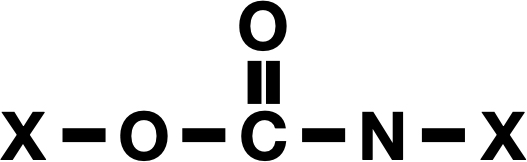
The fundamentals of the chemical bonds that connect polyurethanes. The remaining portion of the polymer is represented by X’s here.
The new study detailing the creation of this enzyme highlights the magnitude of the issue: In 2024, we produced 22 million metric tons of polyurethane. The urethane bond that characterizes these involves a nitrogen atom bonded to a carbon atom that is then connected to two oxygen atoms, one of which integrates into the rest of the polymer. The rest of the polymer, joined by these bonds, can be quite intricate and often consists of ring structures associated with benzene.
Breaking down polyurethanes is difficult. Individual polymer chains are typically heavily cross-linked, and the bulky shapes can hinder enzymes from accessing the bonds they can break down. A substance known as diethylene glycol can partially decompose these molecules, but only at high temperatures. Additionally, it results in a complicated residue of chemicals that cannot be reintegrated into any beneficial reactions. As a result, it is generally incinerated as hazardous waste.